- Retina Research Foundation
- About RRF
- Pilot Study Grants
- Grant Recipients 2025
- Samuel M. Wu, PhD
- Yingbin Fu, PhD
- Graeme Mardon, PhD
- Wei Li, PhD
- Yuqing Huo, MD, PhD
- Rui Chen, PhD
- Wenbo Zhang, PhD
- Curtis Brandt, PhD
- Lih Kuo, PhD
- Timothy Corson, PhD
- Jianhai Du, PhD
- Francesco Giorgianni, PhD
- James Monaghan, PhD
- Seongjin Seo, PhD
- Andrius Kazlauskas, PhD
- Erika D. Eggers, PhD
- Ann C. Morris, PhD
- Ming Zhang, MD, PhD
- Christine Sorenson, PhD
- Alex J. Smith, PhD
- Jeffrey M. Gross, PhD
- David M. Wu, MD, PhD
- Kinga Bujakowska, PhD
- Eric Weh, PhD
- Ching-Kang Jason Chen, PhD
- Jakub K. Famulski, PhD
- Thanh Hoang, PhD
- Georgia Zarkada, MD, PhD
- Eleftherios Paschalis Ilios, PhD
- Oleg Alekseev, MD, PhD
- Erika Tatiana Camacho, PhD
- Patricia R. Taylor, PhD
- Elizabeth Vargis, PhD
- Publications
- Grant Guidelines and Information
- Grant Application
- Grant Recipients 2025
- Research Programs
- Contact Us
- Giving
- RRF History
- Home
Lih Kuo, PhD
Department of Medical Physiology
College of Medicine
Texas A & M Health Science Center
Bryan, TX
GUEYMARD RESEARCH PROJECT
Activation of Endothelin-dependent RhoA/ROCK Pathway Elicits Retinal Microvascular Dysfunction in Diabetic RetinopathyCurrent Research Interests
This project is designed to elucidate the mechanisms responsible for the microvascular pathogenesis of diabetic retinopathy and to develop strategies/tools for the prevention/treatment of this sight-threatening disease. Proper function of the retina depends on an adequate supply of blood (oxygen/nutrients) to the retina, and dysfunction of the retinal microcirculation could lead to disease development. Building upon previous work, Dr. Kuo found that the synthesis of vasoconstrictor/inflammation agent endothelin-1 (ET-1) from endothelin converting enzyme (ECE) is elevated, corresponding to the activation of RhoA kinase (ROCK) and arginase enzymes in the diabetic retina. We hypothesize that ECE/ROCK/arginase signaling contributes to microvascular dysfunction and leads to ischemia for retinopathy development.
Plans for 2025
Dr. Kuo plans to continue his investigation of e the cellular/molecular pathogenesis of diabetic retinopathy, focusing on the induction of retinal microvascular dysfunction by hyperglycemia in association with Retinitis Pigmentosa.
The team will test the sequential activation of the ET-1 system that contributes to the induction of retinal blood flow dysregulation and diabetic retinopathy:
hyperglycemia —->↑ECE/ET-1 —->↑ROCK/arginase —->↑stress kinases —->↑oxidative stress —–> microvasculardys function —-> ischemia/angiogenesis/edema —-> retinopathy.
Additionally, the effectiveness of blocking ET-1 signalings in improving retinal blood flow during early hyperglycemia/diabetes will also be studied.
Specific Aims: Dr. Kuo proposes that hyperglycemia activates the ET-1 system and downstream stress activated kinases (c-Jun N-terminal kinase and p38 kinase), which lead to arginase activation and consequently compromise vasomotor activity and retinal blood flow with a cascade of disease development. We will focus his research on these issues using both pig and mouse models with RP and early type 1 diabetes.
Progress in 2024
The results indicate that early diabetes elicits a significant retinal thinning slightly
before the development of retinal flow reduction in a mouse model of type 1 diabetes. The flow deficiency is associated with impaired endothelium-dependent nitric oxide-mediated vasodilation in ophthalmic arteries feeding to the retinal microcirculation. The changes in vascular reactivity can promote retinal tissue ischemia due to impaired vasodilation and augmented vasoconstriction under hyperglycemic insults. We also found that intravitreal injection of stanniocalcin-1 (STC-1), a secreted peptide displaying multiple regulatory functions in cell survival and death, rescues photoreceptor degeneration with reduced oxidative stress and inflammation in rhodopsin transgenic pigs representing inherited human retinitis pigmentosa (RP). Since the loss of photoreceptor cells is associated with
retinal vascular degeneration in RP, retinal blood flow dysregulation might be related to neural degeneration during diabetes initiation/progression. Treatment of neuropathy and blood flow deficiency in early diabetes by STC-1 may be critical before the establishment of overt neurovascular pathology.
Progress in 2023
The Kuo lab obtained interesting results indicating that early diabetes elicits a significant retinal blood flow reduction with age before the development of pathological retinopathy. This flow deficiency is associated with impaired endothelium-dependent nitric oxide-mediated vasodilation in ophthalmic arteries feeding to the retinal microcirculation. They also found that these ophthalmic vessels exhibited a 100-fold increase in sensitivity to ET-1 with age. These changes in vascular reactivity can promote retinal tissue ischemia due to impaired vasodilation and augmented vasoconstriction under hyperglycemic insults. In contrast to retinal circulation, the blood flow to the brain is normal during the progression of diabetes, suggesting that retinal circulation is more susceptible to diabetic insults. It appears that retinal blood flow dysregulation might lead to neural dysfunction
during diabetes progression and that treatment of blood flow deficiency in early diabetes can be critical before the establishment of overt neurovascular pathology.
Progress in 2022
In 2022, Dr. Kuo’s research yielded three publications with some exciting pilot data. He tested the therapeutic potential of stanniocalcin-1 (STC-1) in rescuing photoreceptor degeneration in a porcine model of retinitis pigmentosa. His lab found that intravitreal injection of STC-1 enhances cone photoreceptor function, improves retinal structural integrity, and reduces retinal degeneration through antioxidative and anti-inflammatory effects. Because diabetic retinopathy is tightly associated with oxidative stress and inflammation in the retina, our findings support that STC-1 might be an excellent drug candidate for treating diabetic retinopathy. The team also demonstrated that retinal blood flow is dysregulated before the development of neural retinal dysfunction in type 1 diabetes. It appears that retinal blood flow dysregulation might lead to neural dysfunction during diabetes progression and that treatment of blood flow deficiency in early diabetes can be critical before the establishment of overt neurovascular pathology.
Progress in 2021
Although Dr. Kuo’s research activity was not fully resumed due to the widespread infection of COVID-19, his laboratory continued their research plan, which yielded three publications. The team documented that activation of stress kinase p38 and sodium-hydrogen exchanger-1 cause enhanced venular constriction to ET-1. Results suggest that treatments targeting these vascular signaling molecules in early diabetes may lessen retinal complications and prevent the development of vascular retinopathy. Dr. Kuo demonstrated that the retinal blood flow is dysregulated before the development of neural retinal dysfunction in type 2 diabetes. It appears that the retinal blood flow dysregulation can lead to neural dysfunction during the progression of diabetes and that treatment of blood flow deficiency in the early stage of diabetes can be critical before the establishment of overt neurovascular pathology.
Dr. Kuo’s 2020 Research Interests
Activation of Endothelin-dependent RhoA/ROCK by C-Reactive Protein Elicits Retinal Arteriolar Dysfunction
This project is designed to bring to light the mechanisms responsible for the microvascular pathogenesis of diabetic retinopathy and to develop strategies and tools for the prevention and treatment of this sight threatening eye disease. Proper function of the retina depends on the sufficient supply of blood to the retina, and dysfunction of the retinal micro-circulation could lead to disease development. in prior research, Dr. Kuo found that the synthesis of vasoconstrictor/inflammation agent endothelin-1 (ET-1) from endothelin converting enzyme (ECE) is elevated, and the RhoA kinase (ROCK) and arginase enzyme are up-regulated in the diabetic retina. We hypothesize that activation of ECE/ROCK/arginase signaling contributes to the retinal microvascular dysfunction and retinopathy. We will use a pig model, which resembles the human eye circulation, to investigate the vascular mechanism and involved signaling pathways in initiation and development of diabetic retinopathy. The pharmacological and molecular strategies will be developed for disease prevention and treatment.
Progress in 2020
Once Dr. Kuo’s research facility resumed activity, his experiments conducted suggested the involvement of reverse-mode sodium-calcium exchanger (NCX) in enhanced retinal venular construction to the inflammatory factor ET-1 during high glucose insults. This abnormality can contribute to retinal edema, a major manifestation of diabetic retinopathy, by elevating capillary filtration and reducing fluid drainage from the venous circulation. Dr. Kuo speculates that the activation of sodium-hyudrogen exchanger-1 (NHE-1), a potential signaling protein upstream of NCX, by stress-activated protein kinases might contribute to venular dysfunction in the diabetic retina.
During the shutdown period, Dr. Kuo’s team focused on submitting manuscripts based on data from the last year, which yielded three (3) publications.
2020 Specific Aims: Dr. Kuo proposes that oxidative stress elicited by activation of the ET-1/ECE system through ROCK/arginase/p-JIP1 signaling leads to vascular dysfunction and that up-regulation of PLv contributes in part to the diabetic angiogenesis elicited by tissue ischemia during hyperglycemic insults. Inhibition of these molecules and signaling pathways is expected to protect and or improve retinal function in diabetes.
Progress in 2019
In 2019, Dr. Kuo’s research team found that retinal venular constriction due to inflammatory agent endothelin-1 (ET-1) is mediated by the ETA receptor activation and Rho kinase (ROCK) signaling independent of protein kinase C. Most importantly, the venular constriction to ET-1 is enhanced in the normal vessels challenged with a hight concentration of sugar solution or in the vessels harvested from diabetic animals (in-vivo hyperglycemia). Results suggest the possible elevation of local capillary pressure and coswequently promotion of fluid filtration and tissue edema, one of the major complications of diabetic retinopathy. Preliminary studies showed that a newly discovered growth factor was up-regulated in human patients with proliferative diabetic retinopathy. Dr. Kuo’s findings were published in peer-review journals.
Progress in 2018
Dr. Kuo’s laboratory found that the inflammatory agent ET-1 causes constriction of retinal venules through calcium entry into the vessel and activation of ROCK enzyme in the vascular wall. Preliminary data indicated that this vasoconstriction is enhanced in the normal vessels challenged with a high concentration of sugar solution or in the vessels harvested from the diabetic animal. Moreover, the team also found that injection of STC-1 to the eye can promote new blood vessel growth in the injured retina due to up-regulation of vascular endothelium growth factor, one of the pathways involved in the development of diabetic retinopathy. Also it was demonstrated that retinal structure and function are susceptible to the alteration of systemic blood pressure per se, suggesting the reduction of blood flow to the eye, as seen in the diabetic subjects, can have an adverse impact on the retinal function. These findings were published in the peer-review journals.
Progress in 2017
In 2017 Dr. Kuo’s research demonstrated that the diabetes-associated inflammatory agent histamine produced dilation of retinal arterioles by releasing vasodilators from the inner wall (endothelium) of the vasculature. Activation of JIP1/JNK signaling during exposure to high glucose or diabetes leads to retinal vascular dysfunction was also found. It appears that diabetic insults selectively impair retinal arteriolar dilation by compromising endothelial function, leading to speculation that histamine might play an important role in increasing microvascular permeability while the endothelial function is compromised by activating endothelial JIP1/JNK signaling pathway in diabetes. In addition, the research team found that eye injections of STC-1 can promote pathological new blood vessel formation by promoting vascular growth-factor signaling. Future effort will be directed to detect whether STC-1 is up-regulated in the diabetic retina and whether administration of STC-1 antibody or antagonists could minimize vessel growth and alleviate diabetic retinopathy. Two papers were published in 2017 in the IOVS and one manuscript under review was later published.
 Progress in 2016
Progress in 2016
Dr. Kuo’s data collected in 2016, was the first to demonstrate the adverse effect of diabetes on retinal microcirculation in the pig model relevant to human physiology and pathophysiology. Recent studies supported by RRF showed that hyperglycemia compromises endothelium-dependent nitric oxide (NO)-mediated vasodilator function in retinal arterioles via increased activation of JNK, a proinflammatory stress kinase. In the pig animal model, he subsequently demonstrated that simvastatin elicits mainly an endothelium-dependent, NO-mediated dilation of retinal arterioles by inhibiting Rho kinase-ROCK pathway. These results strongly suggest that statins have a mechanistic action and therapeutic potential in improving endothelium-dependent vasomotor function in retinal vascular disorders.
Progress in 2015
Dr. Kuo accomplished several projects to elucidate the cellular mechanism responsible for the activation of eNOS by increased flow in association with VEGFR2 signaling. He found that the NO-mediated vasocilation is not compromised by the vasoconstrictor activity of ET-1, and will look for an alternative explanation for the observed clinical outcome of ET-1-asociated retinal disease. The striking result of this year’s study is the involvement of VEGF receptors in mediating flow-induced dilation of retinal arterioles. It appears that VEGFR2 acts as a mechanical sensor for vasodilation to increased flow.
Progress in 2014
Dr. Kuo accomplished Specific Aims focusing on the possible counter-interaction between endothelium-released vasodilator NO and the vasoconstrictor ET-1. The results of this study were published in the Invest Ophthalmol Vis Sci. He further examined the cellular signaling mechanism of activation of NO synthesis by the enzyme NO synthase (NOS) during elevated flow (shear stress). Unfortunately, the mechanism by which NOS is activated by the increased flow for vasodilation remains unknown. The results of this study not only help our understanding on how NOS is activated by flow elevation but also provide useful information on how to protect NOS from the insult of the disease related to retinal ischemia.
Progress in 2013
Dr. Kuo’s project researched the pathophysiology of inflammation and diabetes-associated retinal vascular dysfunction, at molecular, cellular and intact tissue levels to develop a therapeutic approach for disease treatment. He addressed whether cardiovascular risk factors C-reactive protein (CRP) and endothelin-1 (ET-1), in association with oxidative stress, played an adverse role in retinal arteriolar function in diabetes. Dr. Kuo established a pig model of retinal microvascular dysfunction induced by type-1 diabetes, which he showed resembled human retinal vascular physiology and pathophysiology. He continued to utilize the pig model to test central hypothesis that CRP/diabetes activates endothelin converting enzyme activity leading to endothelial dysfunction and impaired vasodilation in retinal arterioles.
Progress in 2012
Dr. Kuo’s laboratory reported that the retinal arteriolar function can be impaired by an acute retinal ischemia due to nitric oxide deficiency and oxidative stress in the endothelium by activating NADP(H) oxidase. This pathophysiology is similar to that elicited by C-reactive protein (CRP) via Rho kinase (ROCK) signaling. In addition they also found that ROCK is a key signaling molecule responsible for the vasoconstriction and oxidative stress evoked by the endothelin-1. Therefore, the endothelin-1 system can be the major linkage between ROCk activation and vascular pathophysiology exerted by inflammation (i.e., CRP) and ischemia. The retinal arteriolar dysfunction can be produced by acute diabetes in the pig, which they have recently shown to resemble human in retinal arteriolar physiology and pathophysiology.
Preliminary data suggest that the retinal vascular dysfunction induced by diabetes might be related to the activation of ROCK via the endothelin system in the vascular wall. This newly developed animal disease model could allow investigation of the early phase of diabetic retinopathy at the mechanistic levels.The purpose of this project is to understand the pathophysiology of inflammation-and ischemia-associated retinal vascular dysfunction at molecular, cellular and intact tissue levels and to develop a therapeutic approach for disease treatment.
Retinal vascular diseases, e.g., diabetic retinopathy, acute angle-closure glaucoma and retinal vascular occlusion are the leading causes of visual impairment in the USA, but the etiology and development of this vasculature-related ocular disease are not fully understood. Because the proper function of retina neuronal tissues depends upon the sufficient oxygen and nutrients supply via the microvascular system, the blood flow regulation by these microvessels is critically important and closely related to the disease development. Early surrogate clinical markers are needed to diagnose and quantitate the presence of preclinical lesions of vascular retinopathy that would allow the institution of treatments at the beginning stages of the disease.
The cellular/molecular mechanisms in regulation of retinal blood flow during disease states remain largely unknown. In this study, Dr. Kuo’s laboratory uses a novel approach (i.e., isolated vessel preparation) to directly address this issue.
The results derived from this study will have significant impact on the design and treatment of vascular disorder in the eye related to inflammatory and ischemia disease. They test the preventive and therapeutic effects of statins, as well as endothelin system blockade and RhoA/ROCK signaling inhibition, on retinal arteriolar dysfunction induced by pathogenic factor CRP/ischema.
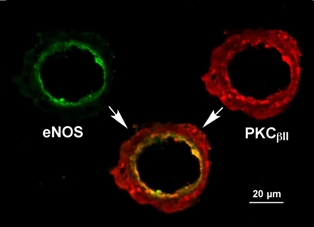 Nitric oxide (NO) released from the vascular endothelium plays an important role in vasodilation, and the deficiency of its synthesis from the enzyme NO synthase (eNOS) contributes to the development of various ocular diseases, including diabetic retinopathy and ischemia-related retinal vascular dysfunction. Although protein kinase C (PKC) activation is also known to participate in the pathogenesis of vascular disease related to ischemia and diabetes, the involved isoform in disease development remains unclear. We investigated the location and distribution of eNOS and PKCβII isoform in porcine retinal arterioles (40-80 µm) using immunohistochemical tools. The eNOS (green) was detected in the endothelial layer of inner arteriolar wall. The PKCβII (red) is located in both smooth muscle and endothelial cells with prominent expression in the endothelium. The close proximity of eNOS and PKCβII in the endothelium may highlight the importance of PKCβII in the regulation of NO production by the endothelium.
Nitric oxide (NO) released from the vascular endothelium plays an important role in vasodilation, and the deficiency of its synthesis from the enzyme NO synthase (eNOS) contributes to the development of various ocular diseases, including diabetic retinopathy and ischemia-related retinal vascular dysfunction. Although protein kinase C (PKC) activation is also known to participate in the pathogenesis of vascular disease related to ischemia and diabetes, the involved isoform in disease development remains unclear. We investigated the location and distribution of eNOS and PKCβII isoform in porcine retinal arterioles (40-80 µm) using immunohistochemical tools. The eNOS (green) was detected in the endothelial layer of inner arteriolar wall. The PKCβII (red) is located in both smooth muscle and endothelial cells with prominent expression in the endothelium. The close proximity of eNOS and PKCβII in the endothelium may highlight the importance of PKCβII in the regulation of NO production by the endothelium.
Progress in 2011
Dr. Kuo’s laboratory developed a clinically relevant animal model for retinal ischemia to support the critical role of ET-1 system and RhoA kinase/ROCK in the development of vascular pathophysiology. The proposed work on the adverse effect of ischemia on retinal arteriolar function was accomplished and results were accepted for publication.
Retinal vascular disease such as diabetic retinopathy is one of the leading causes of blindness in the USA, but the etiology and development of vascular and visual pathology in this disease is not fully understood. Elevated plasma level of inflammatory marker C-reactive protein (CRP) is associated with patients with diabetes and various cardiovascular diseases.
Interestingly, Dr. Kuo’s laboratory found that CRP elicits retinal vascular disorder by losing endothelium-dependent vasodilatory function. However, the mechanistic action of CRP on retinal vasomotor function remained elusive. Since the plasma level of both CRP and endothelin-1 (ET-1), a potent vasoconstrictor, remains elevated in the patients with diabetic retinopathy, the hypothesis that enhanced endothelin-converting enzyme activity (for ET-1 production) and the subsequent RhoA kinase (ROCK) activation are responsible for the adverse action of CRP was tested.
Dr. Kuo also examined the therapeutic potential of statins in the protection and treatment of vascular dysfunction elicited by CRP. His laboratory used an isolated vessel approach to directly assess retinal microvascular function and used molecular tools to address the signaling pathways leading to vascular dysfunction by CRP.


