- Retina Research Foundation
- About RRF
- Pilot Study Grants
- Grant Recipients 2025
- Samuel M. Wu, PhD
- Yingbin Fu, PhD
- Graeme Mardon, PhD
- Wei Li, PhD
- Yuqing Huo, MD, PhD
- Rui Chen, PhD
- Wenbo Zhang, PhD
- Curtis Brandt, PhD
- Lih Kuo, PhD
- Timothy Corson, PhD
- Jianhai Du, PhD
- Francesco Giorgianni, PhD
- James Monaghan, PhD
- Seongjin Seo, PhD
- Andrius Kazlauskas, PhD
- Erika D. Eggers, PhD
- Ann C. Morris, PhD
- Ming Zhang, MD, PhD
- Christine Sorenson, PhD
- Alex J. Smith, PhD
- Jeffrey M. Gross, PhD
- David M. Wu, MD, PhD
- Kinga Bujakowska, PhD
- Eric Weh, PhD
- Ching-Kang Jason Chen, PhD
- Jakub K. Famulski, PhD
- Thanh Hoang, PhD
- Georgia Zarkada, MD, PhD
- Eleftherios Paschalis Ilios, PhD
- Oleg Alekseev, MD, PhD
- Erika Tatiana Camacho, PhD
- Patricia R. Taylor, PhD
- Elizabeth Vargis, PhD
- Publications
- Grant Guidelines and Information
- Grant Application
- Grant Recipients 2025
- Research Programs
- Contact Us
- Giving
- RRF History
- Home
Graeme Mardon, PhD
 Department of Pathology and Immunology
Department of Pathology and Immunology
Baylor College of Medicine
Houston, TX
MILLER RESEARCH PROJECT
Genetic and Molecular Analysis of Retinal DevelopmentCurrent Research Interests
The long-term goal of Dr. Mardon’s research is to improve the collective, clinical ability to prevent, diagnose, and treat human retinal diseases.
The Drosophila eye is a powerful animal model system for deciphering molecular mechanisms of retinal cell fate determination and development. Although our understanding of the molecular genetics of fly eye development is one of the most sophisticated among model organisms, an opportunity for transformative change is presented by the advent of single cell genomics. To that end, we have generated extensive, high quality single nuclear chromatin accessibility sequence (snATAC-seq) data from the normal developing Drosophila eye. In this proposal, we will obtain snATAC-seq data on retinas that are mutant for the onecut gene.
Mammalian homologs of onecut are required for normal retinal development, but the molecular details of how they function are unknown. This new single-cell approach will dramatically increase our understanding of this critical gene family in retinal development. A detailed understanding of the molecular mechanisms of retinal cell fate determination could have broad implications for our ability to diagnose, prevent, and treat human retinal diseases.
From 2011 forward, Dr. Mardon’s laboratory has employed a three-pronged approach. First, they actively map and identify new human retinal disease genes using cutting-edge genomic technologies. Specifically, they have had significant success mapping new genes that cause Leber Congenital Amaurosis (LCA), the most common form of congenital blindness in humans. Second, Dr. Mardon’s laboratory uses the mouse as a model system to study the function of conserved genes required for normal retinal development, including genes identified in their screen of LCA patients. Finally, they use their mouse models to test new treatments to cure blindness, including gene therapy. This combination of approaches comprises an efficient and comprehensive plan to advance understanding of the molecular and genetic mechanisms of human retinal disease.
Plans for 2025
Throughout 2025, Dr. Mardon will continue his studies of the Drosophila onecut gene. Specifically, he will test if the novel targets of onecut discovered in 2024 are required for normal lipid droplet formation and mitochondrial function. These results are likely to provide critical new information about how human homologs of onecut act to control photoreceptor degeneration in humans.
Specific Aims: Dr. Mardon will create animals that are mutant for several onecut target genes, and test if normal lipid droplet formation and mitochondrial function take place. In addition, he will determine if these genes are direct Onecut targets as a test of the hypothesis that Onecut is a master regulator of lipid droplet formation and mitochondrial function.
Progress in 2024
During 2024, Dr. Mardon collected single cell RNA sequence data on onecut mutant retinas, work that he completed and made a major breakthrough concerning this important retinal gene. In particular, he discovered that onecut is required for expression of many genes involved lipid droplet formation and mitochondrial function, two processes known to play important roles in neural degeneration. Moreover, he made complete loss of function mutations in the Drosophila onecut gene and found that these mutants undergo progressive photoreceptor degeneration. This work represents a major step forward in our understanding of basic mechanisms of retinal cell fate determination and is currently being prepared for publication. This project will continue in 2025.
Progress in 2023
In 2023, Dr. Mardon completed the scRNA-seq work on the Drosophila
onecut gene and made a major breakthrough concerning this important
retinal gene. In particular, his research team found that onecut is required for expression of many genes involved axon guidance and synapse formation in developing photoreceptors and this leads to a large disruption in the retina. This work represents a major step forward in our understanding of basic mechanisms of retinal cell fate determination. This project is still in progress and will greatly benefit from continued RRF funding in 2024.
In 2023, Dr. Mardon shifted his laboratory studies to the Drosophila onecut gene. Specifically, his team used single cell genomics to determine the critical targets of onecut activity that are required for normal photoreceptor axon projection to optic lobe. These results are likely to provide critical new information about how human homologs of onecut act to control retinal development and function in humans.
Specific Aims: To obtain single cell RNA sequence data on retinas that are mutant for the onecut gene, which is required for normal retinal development. He will then compare these data to the normal single cell sequence data already obtained from normal developing eyes. This comparison will allow for the determination of the precise molecular defects in onecut mutants and provide important insight into the mechanisms of photoreceptor differentiation.
Progress in 2022
In 2022, Dr. Mardon completed work on the Drosophila rough gene and made a significant breakthrough in his research concerning this important retinal gene. In particular, his lab found that rough is required to repress the senseless gene in developing photoreceptors and this leads to a large disruption in retinal development. This represents a major step forward in the understanding of basic mechanisms of retinal cell fate determination. The project is now complete and will be submitted for publication in 2023.
In 2022, Dr. Mardon shifted studies to Drosophila retinal cell fate determination. Specifically, using single cell genomics to decipher molecular mechanisms of photoreceptor differentiation using Drosophila as a model system.
2022 Specific Aims: To obtain single cell RNA sequence data on retinas that are mutant for the rough gene, which is required for normal retinal development. Subsequently, to compare these data to the normal single cell sequence data already obtained from normal developing eyes. This comparison will allow for determining the precise molecular defects in rough mutants and provide important insight into the mechanisms of photoreceptor differentiation.
Progress in 2021
In 2021, Dr. Mardon completed work on the mouse Spata7 gene and made a significant breakthrough concerning this retinal disease gene. In particular, we found that Spata7 is not only required for the establishment of the connecting cilium in the mouse retina, but it is also required for the maintenance of that structure in adults. This work represents a major step forward in designing therapeutics for inherited blindness. The project is now complete and findings will be submitted for publication in 2022.
Specific Aims: Spata7 encodes a protein that localizes to the base of photoreceptor cells (PRs) and is required for normal trafficking of proteins to the outer segment of PRs where light is transduced into an electric signal. We know that mice lacking Spata7 show clear morphological defects as early as postnatal day 15 (P15) and strong PR loss by P30. However, we do not know if Spata7 is also required for maintenance of PR integrity and function in adults and this has important implications for when and how therapeutics can and should be administered. We propose to remove Spata7 function using a conditional inducible allele and determine if Spata7 is required only for initial CC formation or if it is also required for CC maintenance.
Progress in 2020
In 2020, Dr. Mardon’s team identified a new causative gene associated with congenital blindness, named KCNJ13, which encodes a highly conserved potassium channel protein for which no animal models had been previously established. A detailed understanding of KCNJ13 function in the eye could have broad implications for our ability to diagnose, prevent, and treat human retinal diseases. Plans included further characterization and optimization of the Kcnj13 gene therapy. Specifically Dr. Mardon will use his mouse models to test an experimental viral gene therapy in order to hopefully restore Kcnj13 expression in the retinal pigmented epithelium (RPE), slowdown or prevent retinal degeneration, and maintain the sense of vision in young animals. The goal of the project was to create new reagents that will improve the ability to diagnose and treat human retinal disease, including gene therapy in humans, and this project was completed in 2020.
In 2020 Dr. Mardon completed his work on the mouse Kcnj13 gene and made a significant breakthroughs in his research concerning this retinal disease gene. In particular, his lab found that using AAV-based gene therapy, they were able to significantly rescue the loss of Kcnj13 function in the eye. These results suggest that gene therapy is a feasible approach to treating patients whose have mutations in this gene and therefore represents a major step forward in developing therapeutics for inherited blindness. The project results were submitted for publication in 2021.
Progress in 2019
In 2019, Dr. Mardon continued his study of the mouse Kcnj13 gene and achieved additional research breakthroughs related to this retinal disease gene. His team found that conditional loss of Kcnj13 function specifically in the retinal pigmented epithelium (RPE) in their mouse model leads to early dysfunction of the retina, as early as 15 days old, which ultimately leads to retinal degeneration and blindness. Dysfunction of the retina in the Kcnj13 loss-of-function mouse model coincided with detectable changes in key proteins associated with phototransduction (i.e., light-sensing). The team also observed novel phenotypes in the RPE which led to new conceptual models of how loss of Kcnj13 may lead to retinal disease. Dr. Mardon also began work on designing a viral Kcnj13 gene therapy to test for reduction of disease progression in mice.
Progress in 2018
Throughout 2018 Dr. Mardon’s research concentrated on the mouse Kcnj13 gene and made a significant research breakthrough concerning the retinal disease gene. They found that conditional loss of Kcnj13 function, specifically in the retinal pigmented epithelium (RPE) in the mouse model, caused very early loss of photoreceptors and vision – first detectable within the first 15 days of life. By three months of age, complete loss of the photoreceptor layer was observed. A new system to detect loss of Kcnj13 in the RPE was also developed with which rapid identification of living animals with potential retinal degeneration within minutes – instead of weeks, based on the expression of a genetic biosensor was possible. This mouse model allowed them to dramatically reduce animal usage and study disease progression days before animals have readily detectable vision loss.
2018 Specific Aims: Phenotypic analysis of photoreceptor function in Kcnj13 in mutant retinas. Dr. Mardon proposed to study the role of the gene Kcnj13 in photoreceptor (PR) function and proper expression and localization of key phototransduction proteins. His team hypothesized that loss of Kcnj13 would disrupt PR activity and survival by altering normal PR phototransduction. They used molecular biology and cell biology techniques to measure proteins within the phototransduction pathway.
Progress in 2017
 2017 was exceptionally dynamic for Dr. Mardon’s research. Significantly, his team identified a new causative gene associated with congenital blindness, named KCNJ13, which encodes a highly conserved channel protein for which no animal models had been previously established. A detailed understanding of KCNJ13 function could have broad implications for our ability to diagnose, prevent, and treat human retinal diseases.
2017 was exceptionally dynamic for Dr. Mardon’s research. Significantly, his team identified a new causative gene associated with congenital blindness, named KCNJ13, which encodes a highly conserved channel protein for which no animal models had been previously established. A detailed understanding of KCNJ13 function could have broad implications for our ability to diagnose, prevent, and treat human retinal diseases.
They continued to focus on the mouse Kcnj13 gene and made a significant breakthrough concerning this retinal disease gene. In particular, they found that conditional loss of Kcnj13 function specifically in the retinal pigmented epithelium (RPE) in their mouse model caused very early loss of photoreceptors, first detectable by one month of age. By two or three months of age, they observed complete loss of the PR layer. Armed with that knowledge, they were poised for more detailed studies in the next award year.
Progress in 2016
Dr. Mardon generated a conditional allele of Kcnj13 and demonstrated that loss of Kcnj13 in the RPE causes strong loss of photoreceptors by 3-5 months of age. These data show that his conditional allele is functioning efficiently and that he is now poised for a full developmental study of Kcnj13 function.
Progress in 2015
To create a new mouse model for LCA, Dr. Mardon knocked out the mouse Kcnj13 gene by gene targeting, and analyzed the phenotype of Kcnj13 mutants by histology, immunohistochemistry, electrophysiology, and transmission electron microscopy. In 2015, Dr. Mardon found that conditional loss of Kcnj13 function in his mouse model causes strong loss of photoreceptors, which prepared him for detailed studies in the next award year.
Progress in 2014
Dr. Mardon made a significant breakthrough in his research concerning the Kcnj13 retinal disease gene. Specifically, his laboratory found that loss of Kcnj13 function in their mouse models causes strong loss of photoreceptors and he began full-scale analysis in the next award year. Dr. Mardon generated and characterized null and conditional mutations in this critical human disease gene in mice and has shown that homozygous mutant mice recapitulate the human disease phenotype.
Progress in 2013
Progress in Dr. Mardon’s laboratory took an exciting turn in 2013 as his team identified a new gene associated with LCA (named KCNJ13), which encodes an inwardly rectifying potassium channel but for which no animal models had been established. His preliminary evidence suggested that his mouse mutation may be homozygous lethal. Therefore, in addition to characterizing this allele in more detail, he worked to also generate conditional alleles of Kcnj13. A detailed understanding of Kcnj13 function could have broad implications for our ability to diagnose, prevent, and treat retinal diseases.
Progress in 2012
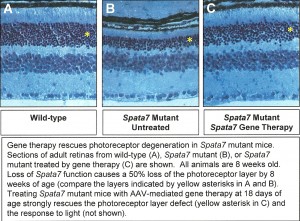
To create a new mouse model for LCA, Dr. Mardon has knocked out the mouse SPATA7 gene by gene targeting. SPATA7 mutants are homozygous viable but have severe defects in retinal development, closely mimicking the human disease. Dr. Mardon has analyzed the phenotype of SPATA7 mutants by histology, immunohistochemistry, electrophysiology, and transmission electron microscopy. A Manuscript is being prepared reporting this work. Dr. Mardon is now making rapid progress using this model to develop gene therapy approaches with the ultimate goal of treating human patients with mutations in SPATA7.
Dr. Mardon’s laboratory identified the causative gene associated with LCA3, named SPATA7, which encodes a highly conserved but novel protein of unknown function and for which no animal models had been established. Significantly, SPATA7 mutations are associated with both LCA and early-onset retinitis pigmentosa (RP), suggesting that a detailed understanding of SPATA7 function could have broad implications for diagnosis, prevention, and treatment of human retinal diseases.
In 2012, one of the most promising methods for treating human retinal disease emerging was the use of gene therapy. Dr. Mardon used the mouse model of LCA that his laboratory developed in 2011 as a system for testing the efficacy of gene therapy.
Specifically, they tested how long after disease symptoms appear could mice be treated by gene therapy while still showing significant improvement in visual function. They used the adeno-associated virus (AAV) system to restore Spata7 gene function specifically in the eyes of Spata7 mutant mice. They tested for response to light using electroretinograms. Dr. Mardon also tested for restoration of photoreceptor cells using histology and light microscopy. Data from these experiments proved to have important implications for human gene therapy approaches in the future.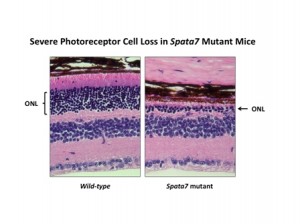
By one year of age, Spata7 mutant mice present with a severe loss of photoreceptors, as seen by the large reduction in the outer nuclear layer (ONL) of the retina. These mice fully recapitulate the human LCA disease phenotype and are now being used for gene therapy studies.
Progress in 2011
Dr. Mardon’s laboratory used a mouse model they created for LCA3 to decipher the mechanism of disease in humans and to validate the model for gene therapy studies. They successfully completed their goals, documenting that severe reduction in the number of photoreceptor cells and little or no response to light in the absence of Spata7 function was due to the mislocalization of the visual pigment Rhodopsin.
Normally, virtually all Rhodopsin protein is found in the outer segment of photoreceptors. In Spata7 mutants, Rhodopsin was found throughout the cells, which then later died. If both Spata7 and Rhodopsin were removed, cell death was blocked, demonstrating that mislocalization of Rhodopsin is the key event leading to photoreceptor loss. This model was validated for the next major goal: to develop gene therapy approaches.